The problem
Full preservation of the two sets of nerves and blood vessels (the neurovascular bundle or NVB) that lie at the back of the prostate is the most important step in maintaining potency (the ability to have penetrative intercourse) following robotic prostatectomy, but this step frequently results in some oozing of blood due to there being lots of small blood vessels in the area. This ooze usually stops on its own, but in less than 1% of patients it continues and may cause pain, infection, tension on the new join between the bladder and the urethra (where it is stitched together following the removal of the prostate) and rarely (1 in 400 patients) requires a blood transfusion. Using cautery (heat), stitches and small titanium clips to reduce this ooze is undesirable as these steps can interfere with the function of the NVB and lower potency rates.
A potential solution
Several haemostats (solid or liquid products that reduce bleeding) are available for use during surgery, but some are known to cause inflammation, scarring and interference with post-operative scans (which are sometimes needed if there is cancer recurrence in high-risk cancer patients). However, we have been using a new haemostat for the last several years which has proven to be highly effective.
Arista is a powdered haemostat that is easy to apply robotically through a flexible plastic tube, is plant-derived and disappears completely from the body within 48 hours. We have now used Arista for all 971 of our most recent patients and analysis of our early results showed that it did indeed reduce bleeding-related complications following robotic prostatectomy, but patients treated with Arista also surprisingly had better potency rates. These results have been published and presented internationally.
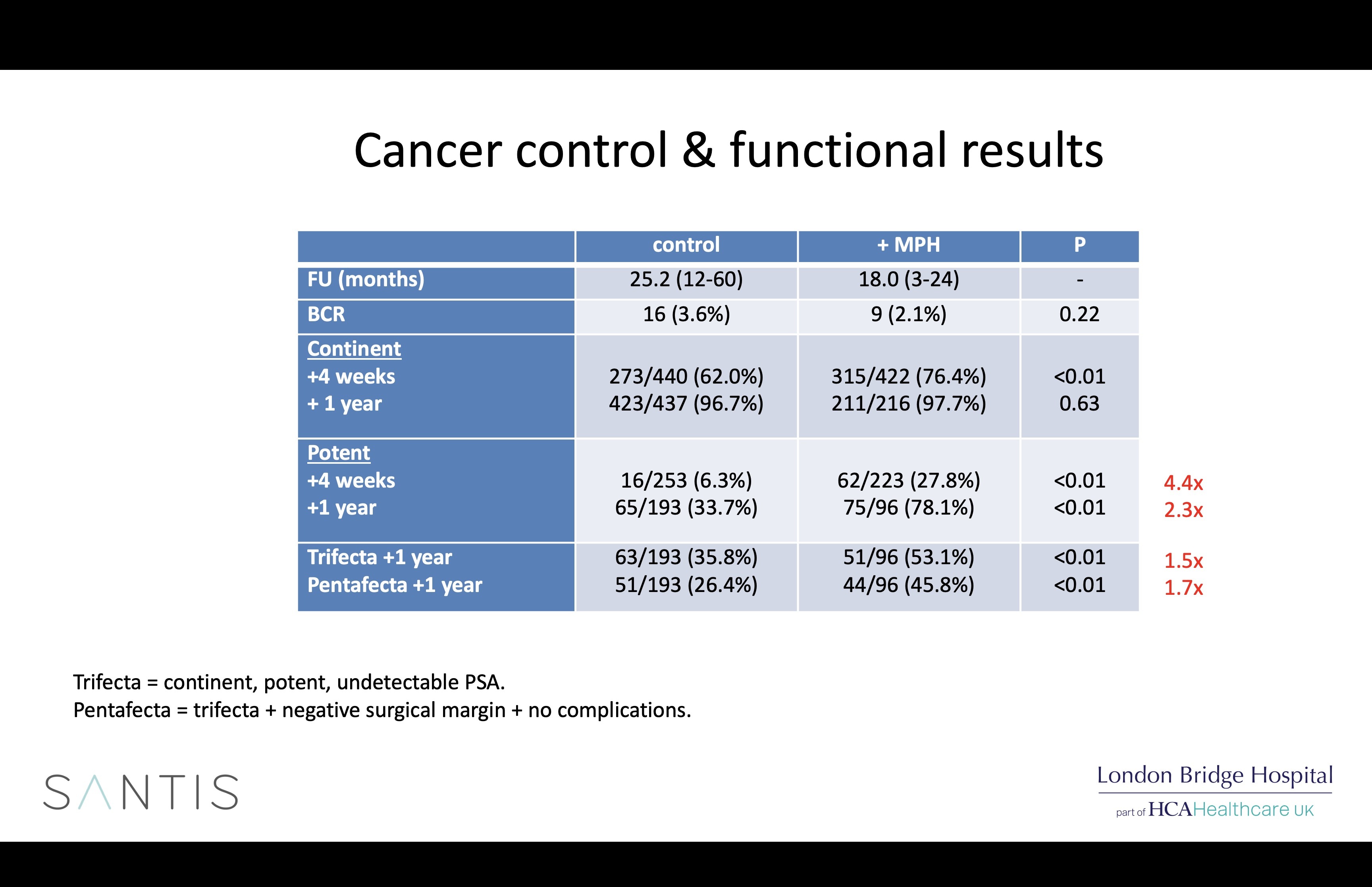
Possible explanations for the differences seen in the 2 groups (which were run one after the other) include greater surgical experience, better nerve preservation due to the adoption of a new early posterior release technique adopted 6 years ago, and Arista, possibly by coating the NVBs with a gelatin matrix which protects them from the harmful effects of inflammation or, most likely, all of the above.
To definitively answer this question, we plan a simultaneous randomised controlled trial in 2023 with two other high-volume European centres (Hamburg and Gronau) to investigate these differences. Whatever the results of the trial, they will certainly be interesting and we will post them here!

